Abstract
Background: Response in acute myeloid leukemia (AML) is based on morphologic evaluation of bone marrow (BM) and peripheral blood lab-based assessments. Electronic health records (EHRs) hold structured information on peripheral blood assessments, but it may be incomplete; unstructured documents containing BM morphologic evaluations can be abstracted to derive data on morphologic leukemia-free state (MLFS). The objective of this analysis was to assess the feasibility of capturing MLFS using EHR with enough completeness for analytic use. The association of MLFS status with real-world time to next treatment (rwTTNT) and real-world overall survival (rwOS) was evaluated.
Methods: This analysis utilized data from the nationwide Flatiron Health EHR-derived de-identified database and included 2,560 patients diagnosed with AML who initiated first line therapy(1L) with either 7+3 regimen or a hypomethylating agent (HMA) + venetoclax between January 1, 2014 and February 28, 2021; with follow up through May 31, 2021. Evidence of MLFS using abstracted data from BM biopsies was defined as ≤ 5% blasts in the BM, no Auer rods, and no extramedullary disease. Patients' first BM biopsy in the EHR that occurred between 14 to 60 days after 1L initiation were used to assess MLFS in investigations of rwTTNT and rwOS, regardless of MLFS completeness. The Kaplan-Meier method was used to evaluate the association of MLFS with rwTTNT and rwOS landmarked at 60 days after 1L initiation to account for differences in time between 1L initiation and MLFS assessment.
Results: Among the full cohort, 2,142 (84%) patients had a BM biopsy at any point during 1L. The median and mean number of biopsies in the EHR per patient that occurred during 1L were 2 and 2.5 (SD: 1.6), respectively, with a range of 1 to 13 biopsies per patient. There were 5,328 BM biopsies across all patients during 1L, and complete information was available to derive MLFS status for 4,584 (86%) biopsies. Blast percentage was the most frequently missing component. There were 1,811 (70%) patients who had a BM biopsy between 14 and 60 days after 1L initiation. There were 2,589 BM biopsies across all patients between 14 and 60 days after 1L initiation, and complete information was available to derive MLFS status for 2,259 (87%) of biopsies. All 1,811 patients with a BM biopsy between 14 and 60 days after 1L initiation had complete information to ascertain MLFS status on at least one biopsy. The median time between 1L initiation and the first BM biopsy that occurred at least 14 days after 1L initiation was 44 days.
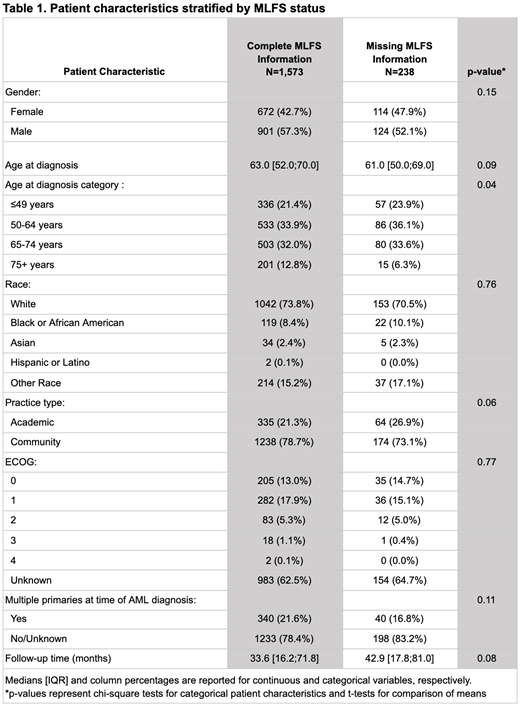
There were 1,573 patients (with BM biopsy in 14-60 days post 1L) included in analyses of rwTTNT and rwOS (238 patients were excluded due to incomplete information on MLFS components on their first BM biopsy). Patients without complete information to derive MLFS status were less likely to be an advanced age at diagnosis (75+), more likely to be seen at an academic practice, and had longer average follow-up (Table 1). Among patients with complete information to derive MLFS status, 1,123 patients had evidence of MLFS and 450 patients had no evidence of achieving MLFS. When landmarked at 60 days after 1L initiation, median rwTTNT was 5.8 months (95% CI: 4.8, 7.9) and 3.4 months (95% CI: 2.6, 4.1) for patients with and without evidence of MLFS, respectively. Median rwOS landmarked at 60 days after 1L initiation showed a similar trend, with a median rwOS of 26.9 months (95% CI: 19.4, 40.6) and 12.5 months (95% CI: 11.1, 15.5) for patients with and without evidence of MLFS, respectively.
Conclusion: Bone marrow biopsies were available during 1L for a high proportion of the study population. Similarly, components to derive MLFS status were available for the large majority of biopsies. When focusing on biopsies occurring between 14 to 60 days after 1L initiation, complete information to ascertain MLFS status was available for at least one BM among all patients. In analyses with analytic endpoints, MLFS status was associated with longer rwTTNT and rwOS, in line with study hypotheses. Together, this information indicates a high degree of completeness in deriving MLFS using EHR data that may be used for analytic purposes.
Guinter: Flatiron Health, Inc.: Current Employment; Roche: Current equity holder in publicly-traded company. Kim: Flatiron Health, Inc.: Current Employment; Roche: Current equity holder in publicly-traded company. Jacob: Flatiron Health, Inc.: Current Employment; Roche: Current equity holder in publicly-traded company. Wise: Flatiron Health, Inc.: Current Employment; Roche: Current equity holder in publicly-traded company. Cha: Flatiron Health, Inc.: Current Employment; Roche: Current equity holder in publicly-traded company. Sawas: Affimed: Research Funding; Roche: Current equity holder in publicly-traded company; Flat Iron Health: Current Employment; Seattle Genetics: Honoraria; Gilead: Speakers Bureau; Acrotech: Honoraria; Daiichi-Sankyo: Speakers Bureau; Seattle Genetics: Speakers Bureau.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal